GLP-1 MEDICATIONS
Retatrutide: New Kid on the Block
We are getting lots of questions about retatrutide, the “new kid on the block” in the weight‑loss and metabolic‑health space. It is a once‑weekly injectable that targets three hormone pathways at once to reduce appetite, improve blood sugar, and reshape metabolism. But, it is actually not even actually on the block yet. It is not yet FDA‑approved. Most experts expect that if results remain strong, the earliest realistic U.S. approval window would be around 2026–2027.
Does it work? In phase 2 and phase 3 studies, adults without diabetes have been shown to lose 20-30% of their body weight when using retatrutide. For fatty liver disease, retatrutide has shown particularly striking effects: 80–85% of patients on higher doses achieved near‑complete clearing of liver fat. See the article below with additional content online for a more in-depth discussion.
GLP-1s: Summarizing the Research
GLP-1–related medications have quickly moved from being “just diabetes drugs” to becoming central tools for weight loss and long-term heart, liver, and kidney health, but the strength of evidence is not the same for every use. This overview keeps the language accessible while still reflecting where the science is strongest (semaglutide, tirzepatide, retatrutide) and where it is early and experimental (microdosing for inflammation and autoimmunity).
How these medications work
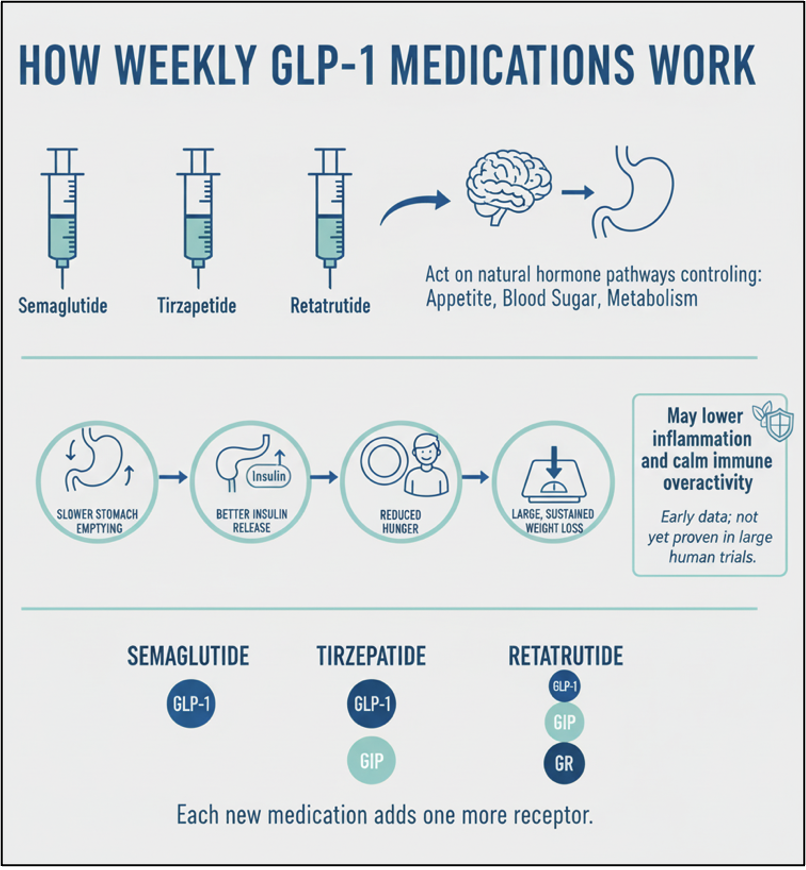
Semaglutide, tirzepatide, and retatrutide are weekly injectable medications that act on natural hormone pathways in the gut and brain that control appetite, blood sugar, and metabolism. They slow how quickly food leaves the stomach, help the pancreas release insulin more effectively, reduce hunger, and support large and sustained weight loss. Laboratory and early human studies also show that this hormone pathway can lower levels of inflammatory chemicals in the body and calm down overactive immune responses, suggesting possible benefits for chronic inflammation and autoimmune conditions, though this is not yet proven in large humans trials. However, we have seen these benefits for some of our patients at Paradigm.
These medications work by effecting 3 different types of receptors in the body: GLP-1 (glucagon-like peptide-1), GIP (glucose-dependent insulinotropic polypeptide), and GR (glucagon receptor agonist). Each new medication adds one more receptor.
Weight, metabolism, and whole‑body health
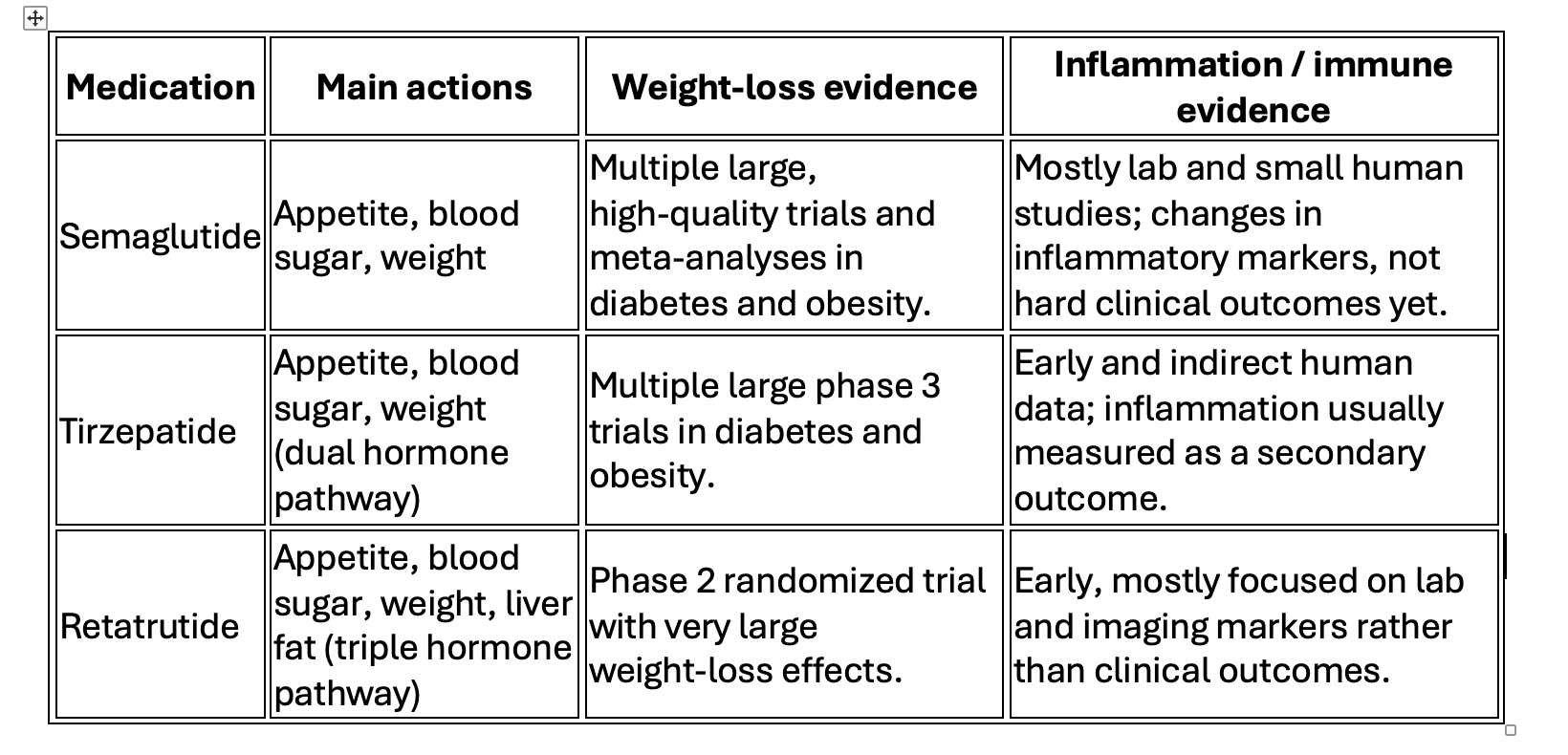
For people with obesity and type 2 diabetes, semaglutide and tirzepatide have very strong evidence from large, high‑quality clinical trials showing meaningful weight loss, better blood sugar control, and improvements in blood pressure, cholesterol, and markers of inflammation. Retatrutide, which is still in earlier stages of research, has shown some of the most powerful weight‑loss effects seen so far in a phase 2 trial, with average losses over 20% of body weight at higher doses, but long‑term safety and durability are still being studied. Many of the benefits for heart, liver, and kidney health appear to come from this combination of weight reduction, better blood sugar, lower blood pressure, and improved cholesterol and triglycerides.
Heart and kidney protection
For the heart, semaglutide and tirzepatide in this family have been tested in large, long‑term trials in people with type 2 diabetes and high cardiovascular risk, showing a clear reduction in major events like heart attack, stroke, and cardiovascular death. Kidney benefits are also emerging: both semaglutide and tirzepatide improve urine protein levels and slow the decline in kidney function in people with diabetes, with these effects likely explained partly by weight and blood pressure changes and partly by direct kidney effects.
Liver health and fatty liver disease
In people with fatty liver related to metabolic health, semaglutide and tirzepatide lower liver fat on imaging and improve liver enzymes in randomized trials, although complete reversal of scarring is less consistently shown and still under active study. Retatrutide has produced particularly impressive results in early trials, where more than 80–85% of patients at higher doses reduced liver fat to normal levels on MRI, effectively clearing visible fat from the liver in many participants; this is strong early evidence, but longer follow‑up is needed to know what it means for fibrosis and long‑term outcomes. These liver improvements track closely with better weight, insulin sensitivity, triglycerides, and abdominal fat, supporting the view that these medications help “reset” the broader metabolic environment.
Microdosing, inflammation, and autoimmunity
“Microdosing” GLP‑1–type medications—using much smaller, more frequent doses with the goal of calming inflammation while limiting side effects—is an emerging off‑label approach in concierge and integrative practices. Research in cells, animals, and small human studies shows that this class can reduce inflammatory signals, affect immune cell behavior, and possibly influence brain–immune communication, which makes it biologically plausible as an adjunct strategy in autoimmune and chronic inflammatory diseases, but current human data are limited to case reports, small series, and observational experiences rather than rigorous controlled trials. That being said, we have found tirzepatide to appear to have benefit for these conditions in some of our patients.
How this fits a longevity‑focused practice
For a practice focused on healthspan and longevity, semaglutide and tirzepatide have the strongest support as core tools for weight management, diabetes control, and reduction of heart and kidney risk in carefully selected patients, based on multiple large randomized trials and pooled analyses. Retatrutide represents a powerful next‑wave option, especially for severe obesity and fatty liver, but is still in earlier phases of study, so its use requires more caution and close monitoring until phase 3 and outcomes data are available. For autoimmune and inflammatory indications—especially microdosed protocols—the science is at an early, hypothesis‑generating stage, and the most responsible approach is to present these strategies as promising adjuncts grounded in biology and early clinical experience, not as proven replacements for established therapies.
Using All the Tools in the Toolbox
Healthy, sustainable weight loss rests on four pillars: sleep, diet, exercise, and—when appropriate—GLP‑1–based medications. Human clinical trials confirm that each pillar plays a distinct role, and that combining them produces better and more durable results than relying on any single strategy alone.
Sleep: the hidden metabolic lever
Randomized and longitudinal human studies show that short or poor‑quality sleep increases hunger hormones, drives higher calorie intake, and predicts weight regain after initial weight loss. In a 1‑year trial of adults with obesity, those with shorter sleep after an initial low‑calorie diet regained more weight and lost less fat than normal sleepers, even when given exercise and liraglutide. Extending sleep in people who are habitually sleep‑deprived reduces free‑living calorie intake and promotes a negative energy balance, directly supporting weight loss and maintenance.
Diet: quality and calorie control
Calorie reduction with adequate nutrition consistently produces weight loss and cardiometabolic improvements in randomized human trials. Long‑term calorie‑restriction studies show sustained reductions in body weight, fat mass, fasting glucose, and lipids, with preserved or improved diet quality, supporting calorie‑controlled, nutrient‑dense, high-protein eating as a safe and effective strategy for healthspan. Importantly, lasting behavior change around food—rather than short, extreme diets—predicts better long‑term maintenance.
Exercise: cardio plus resistance
Exercise trials in adults with excess weight demonstrate that aerobic training is particularly effective for reducing fat mass and body weight, while resistance training is essential for increasing or preserving lean muscle. Combined aerobic plus resistance programs reduce body fat, improve metabolic syndrome markers, and increase lean mass more robustly than usual care, confirming that pairing “cardio” with strength work is metabolically superior to either alone for many patients. This combination directly supports the goal of losing fat while building or preserving muscle, which enhances insulin sensitivity, functional capacity, and long‑term metabolic health.
GLP‑1 medications: powerful, but not standalone
As noted above, GLP‑1–based medications produce substantial weight loss and cardiometabolic benefits and are valuable tools when lifestyle changes alone are insufficient. However, data from several trials show that the combination of medications plus diet and exercise (specifically resistance training) are needed for optimal outcomes: weight loss with muscle preservation.
Taken together, human trial data strongly support the four‑pillar model: adequate sleep, calorie‑ and quality‑conscious nutrition, combined aerobic and resistance training, and GLP‑1–based medications, when indicated, form a coherent, evidence‑based framework for healthy weight loss and long‑term metabolic resilience.